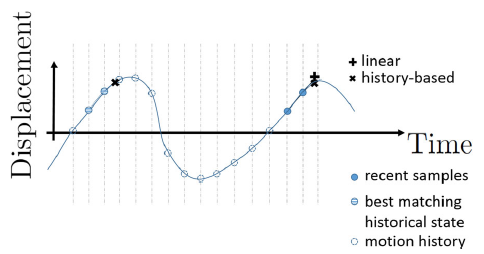
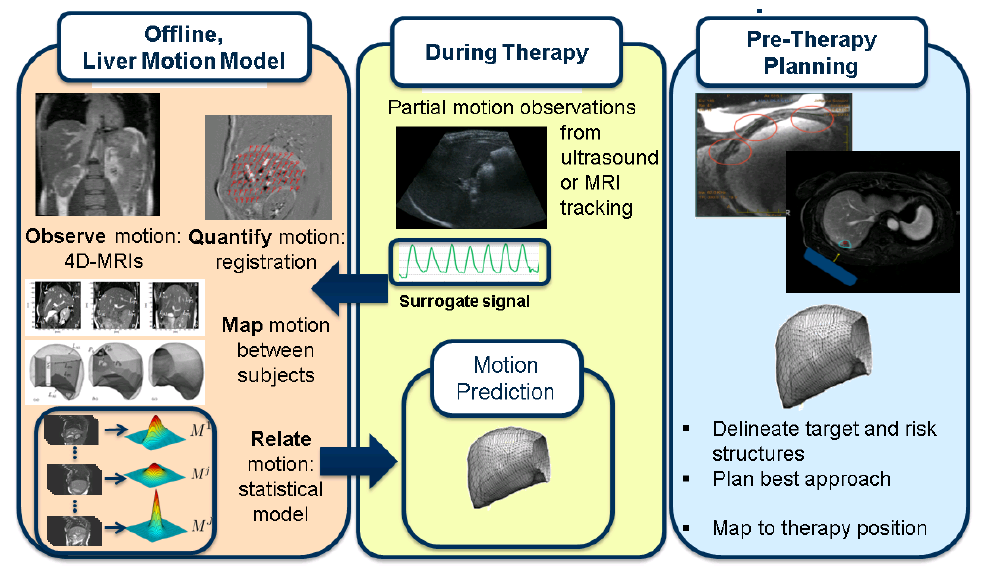
Overview of TTS
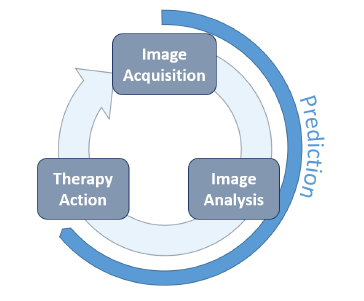
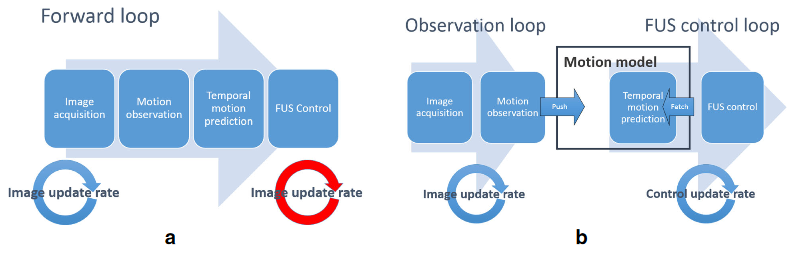
The TRANS-FUSIMO treatment software (TTS) is one of the integral and essential developments during the TRANS-FUSIMO project. Only with the TTS, a focused ultrasound treatment in moving abdominal organs becomes feasible. To our knowledge, this system is unique in the research community. No other group of researchers has been able to integrate the necessary components into a real-time steering system that is extensively validated. The system is implemented as a generic treatment system that abstracts from actual vendor-specific hardware detail. MRgFUS is an image guided therapy (see above figure), thus, the system continuously requires images as input in order to extract information for adjustments of the therapy in real time. In the TRANS-FUSIMO case, images are acquired with a GE MRI device. This data is then processed and the resulting information leads to a therapy action which is the update of the FUS device.
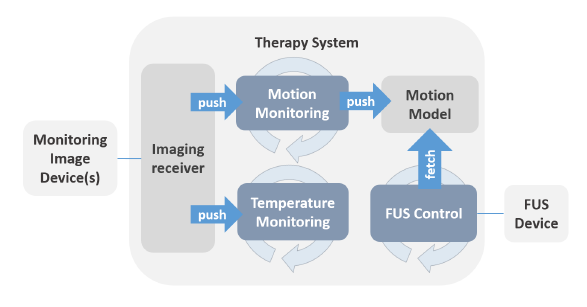
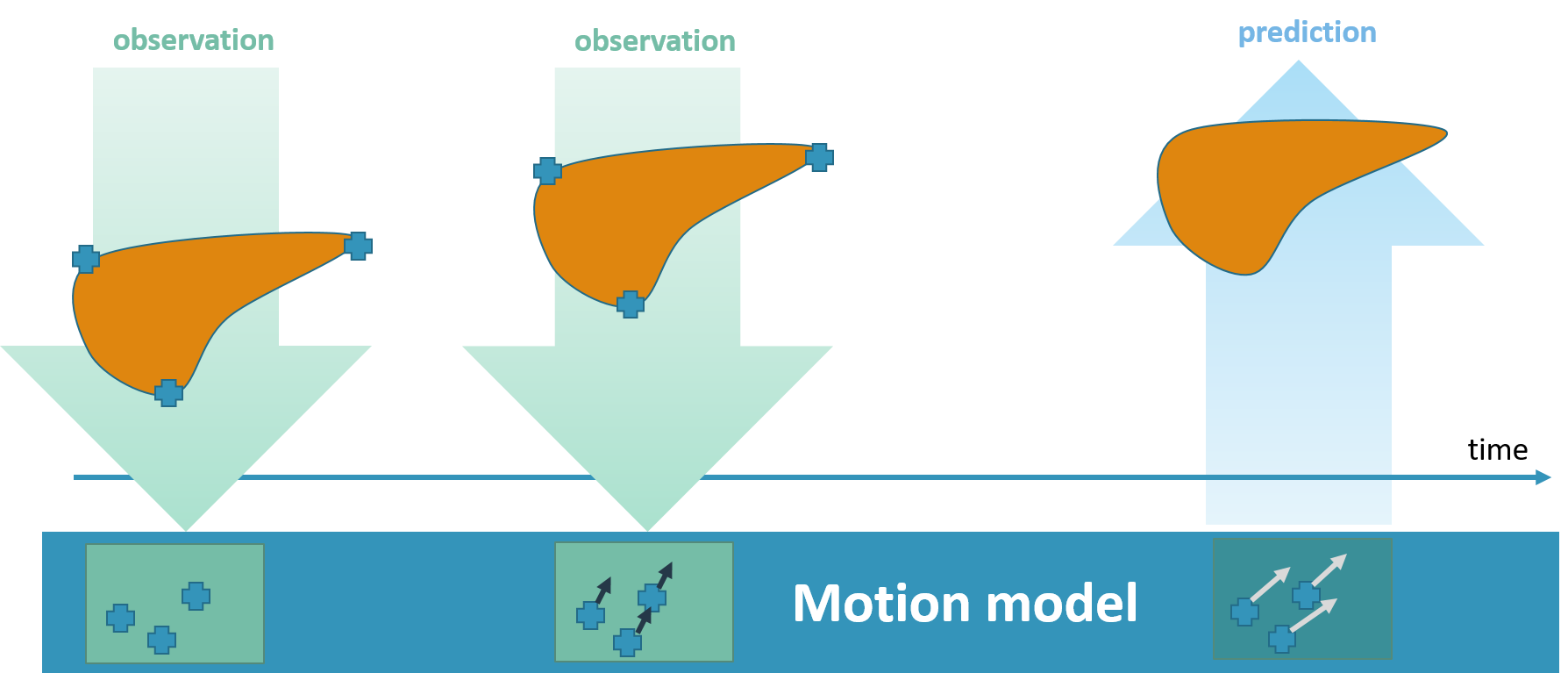
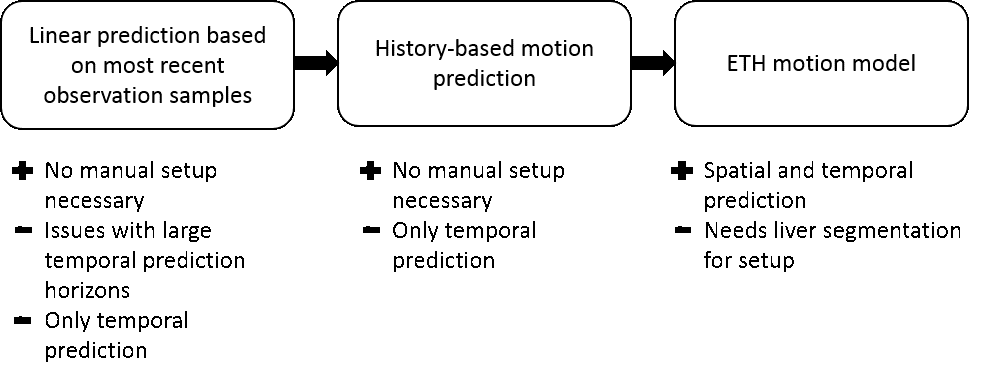
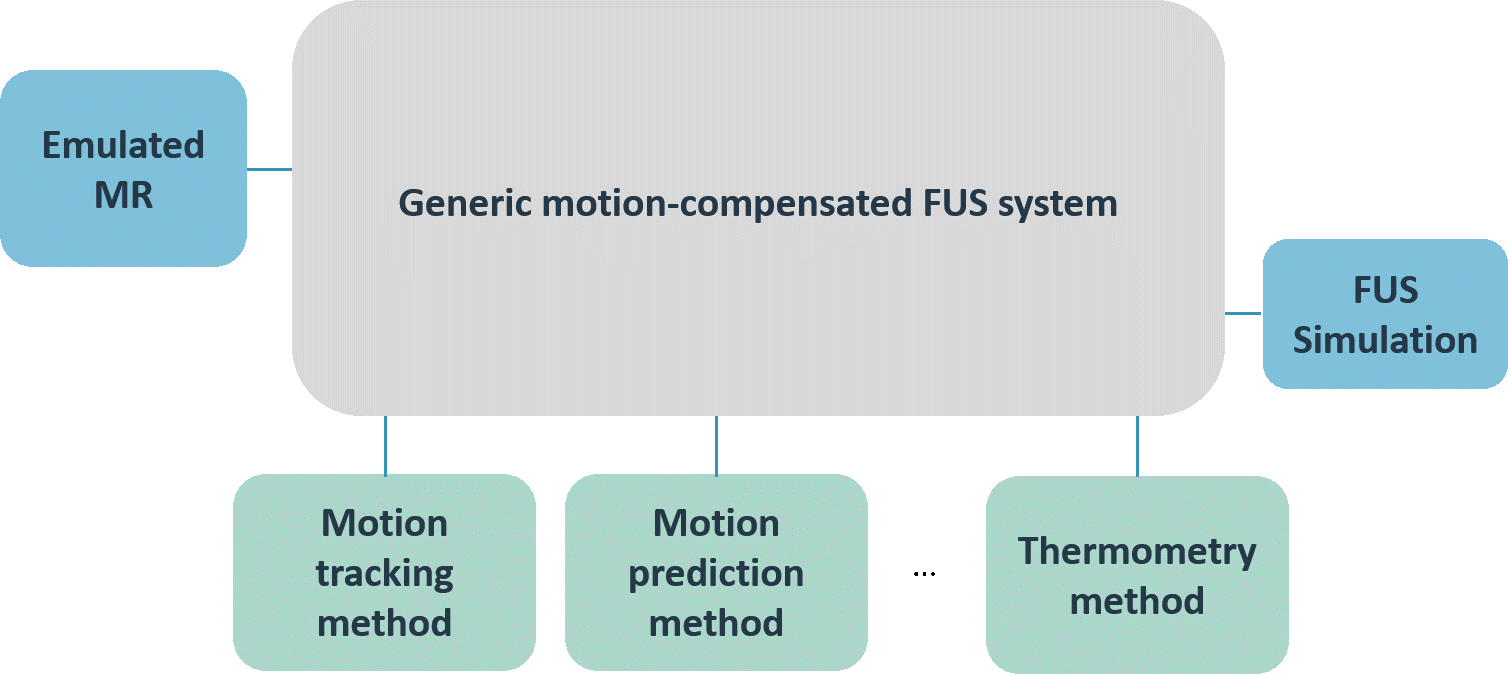
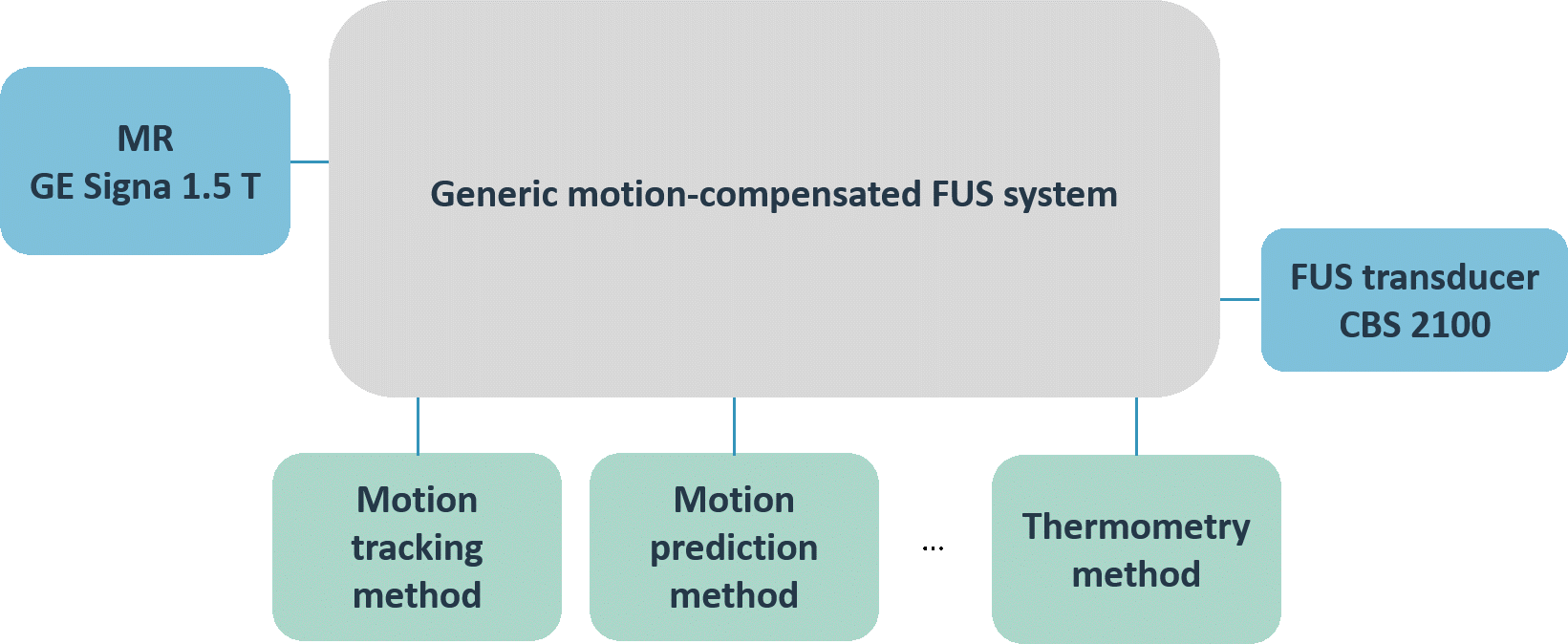
We aimed at developing a generic motion-compensated FUS system that is independent of any algorithmic implementations needed for the real-time processing pipelines or hardware specific details. This generic system takes care of all the incoming information and data and handles the core functionality. The grey box in the left middle image visualizes the so-called core part of the software. The implementations of the algorithms can be added like plug-ins that, however, are mandatory for the full functionality of the software. The green boxes in the same figure depict the algorithms needed for the motion compensation and monitoring whereas the blue boxes represent the connection to hardware.
In a next step, we implemented the FUS control of the physical transducer CBS 2100 of INSIGHTEC as well as the imaging receiver for the MRI device of GE. The lower figure on the left shows the specific hardware implementations of the generic FUS system. This specific implementation of the generic motion-compensated FUS system was used for all pre-clinical experiments performed during TRANS-FUSIMO.
 Fraunhofer Institute for Digital Medicine MEVIS
Fraunhofer Institute for Digital Medicine MEVIS